A biomedical tale linked to the Academy of Sciences of Bologna Institute
Lucio Ildebrando Maria Cocco
University of Bologna, Medical School and Academy of Sciences of Bologna Institute;
Benedictine Academician, Perpetual Secretary
Abstract
In this review, I discuss the extraordinary achievement in the field of morphology and medicine by Marcello Malpighi. It is my intent to underline how it was difficult for Malpighi to obtain recognition of his great discoveries. Then I report the hard steps faced by a research team in Bologna, that I have joined since late seventies. We were able to demonstrate the existence inside the nucleus of an autonomous system of signalling based on peculiar phospholipids, i.e. inositol lipids.
Introduction
As I began to write this story it occurred to me how difficult it is to get new ideas accepted in the scientific world and how the “power” of some can influence the fate of important discoveries. This chapter for the Annales of the Academy tells the story of the discovery and clinical fallout by Academics of our Academy of a new morpho-functional “world” in the nucleus and the great discoveries of a “precursor” and Master of the founders of the Academy, Marcello Malpighi. The things in common? Both Malpighi (https://it.wikipedia.org/wiki/Marcello_Malpighi) and the two Academic discoverers of the “new world” in the nucleus, Francesco Antonio Manzoli (https://it.wikipedia.org/wiki/Francesco_Antonio_Manzoli) and the author of this article (https://it.wikipedia.org/wiki/Lucio_Ildebrando_Cocco) can be found on Wikipedia, and all had to “fight” against preconceived ideas in their respective times.
1. Malpighi and his seminal work
To be historically precise, Malpighi is not among the Benedictine Academics simply because his death in 1694 preceded the foundation of the Academy, in 1711, by Count Luigi Ferdinando Marsili, who, however, drank abundantly from Malpighi’s fountain of science.
The young Malpighi rejected the theoretical ideas, dominant at that time, still tied to a philosophical/Galenic and astrological conception of medicine, drawn instead to a new scientific awareness of the discipline. In a university like that of Bologna, which was not up-to-date with European innovations, these revolutionary positions cost Malpighi, making him the target of heated accusations and criticism from his fellow students as well as professors, such as Tommaso Sbaraglia, Agostino Cecchi and Ovidio Montalbano, who did everything in their power to obstruct the conferral of his degree and, more generally, the experiments carried out by the “anatomical chorus”, an academic group that met at the home of the physician and professor Bartolomeo Massaro.
Around the age of 38, and with a remarkable academic career behind him, Malpighi decided to dedicate his free time to anatomical studies. Although he conducted some of his studies using vivisection and others through the dissection of corpses, his most illustrative efforts appear to have been based on the use of the microscope. Because of this work, many microscopic anatomical structures are named after Malpighi, including a skin layer (Malpighi layer) and two different Malpighian corpuscles in the kidneys and the spleen, as well as the Malpighian tubules in the excretory system of insects.
With the publication of his two “Epistle de Polonius” in 1661, he once again found himself at the centre of criticism from his Galenic rivals. Illustrating the structure of an organ for the first time, thanks to his use of a microscope, he was able to debunk the theory that the lungs were formed of coagulated blood, through which the pneuma was given to the heart. Malpighi was able to instead grasp the hollow and alveolar nature of the organ and hence hypothesise the respiratory theory. In the second publication, by studying the lungs of frogs, he established the circulatory nature of the blood vessels, already guessed in 1628 by Harvey [1].
The endless harassment drove Malpighi to move to different universities, Pisa, Messina, but he always came back to Bologna. But… in 1683, a fire destroyed Malpighi’s home in Corticella, burning all of his writings and expensive work equipment (some believe that the fire was started by Giovanni Girolamo Sbaraglia and his followers).
In spite of this irreparable damage, the Royal Society, that co-opted him as a foreign member, published his Opera omnia in 1686 (Fig. 1) cementing the international arrival of the Bolognese scientist. I am keen to underline the importance of the fact that the Royal Society made an international recognition for his discoveries. Could be true the ancient latin line “nemo propheta in patria”?

Fig. 1. Cover of Opera Omnia (Complete Works), London 1686. Published (in Latin) by the Royal Society.
Despite his wealth and fame, he continued to be harassed, even within the university, by first-rate detractors, including the botanist Giovani Battista Trinetta, the physician Giovanni Girolamo Sbaraglia, the chancellor and archdeacon Anton Felice Marsili and even some of Malpighi’s own students. Albeit the Royal Society made international recognition also in Italy he had support by the papal legate Antonio Pignatelli who, after becoming Pope Innocent XII in 1691, convinced the now elderly Malpighi, his personal doctor, to come to Rome as his chief physician.
In summary Malpighi was a pioneer in the morpho-functional disciplines. His seminal work paved the way for the modern medicine, but the “scientific” or I should say “political” power was against him in his beloved city. Thank God the Royal Society and Pope Innocent XII gave him full recognition of his discoveries.
Today, the memory of the great scientist is more alive than ever in the city that loved him so deeply but also did so much to obstruct his work. At Palazzo dell’Archiginnasio, the portrait painted of him by the classicist Marcantonio Franceschini in 1683-87 is a luminous, joyful counterbalance to the portrait made shortly after by Donato Creti of his antagonist Giovanni Girolamo Sbaraglia, portrayed melancholic and lost in thought. At the end of this part of the tale we must take into account that whilst Sbaraglia, the most powerful detractor of Malpighi, is a completely unknown name, that of Malpighi will remain imperishable over time.
2. The discovery of nuclear inositol lipid signalling
In the sixties it was shown that the phospholipid content of active chromatin was 5-fold higher compared to repressed chromatin [2]. Professor Manzoli and his lab mate Cocco were interested in pursuing the biological significance of this observation with regards to cell cycle regulation, but the stumbling block was persisting controversy over the extent to which these phospholipids might arise by contamination from non-nuclear membranes. Manzoli and Cocco investigated this problem, and they concluded that non-contaminating phospholipids were genuinely associated with non-histone chromosomal proteins (NCHP), perhaps regulating DNA stability and replication. They also reported that NHCP fractions prepared from leukemic B-lymphocytes contained 40% less sphingomyelin and 4-fold higher levels of phosphatidylcholine, compared to normal B-cells [3].
But the persisting controversy over the real existence of nuclear phospholipids was still strong. A good way to jump over the persisting controversy seemed to investigate if there could be a role for nuclear phospholipids. Indeed in 1980 a paper from Gilmour laboratory in Glasgow and Manzoli and Cocco laboratory in Bologna described the analysis of Triton-treated nuclear material to remove nuclear membranes [4]. This short paper presented an important finding that put-to-bed the criticism that phospholipids in nuclear preparations must have arisen from contaminating membranes. Indeed data had shown nuclear phospholipids need not even be associated with membranes. But still a bit of skepticism remained. That was the time of the emergence of inositol lipids as cellular signals [5]. They are present at very low levels and at that time were canonical plasma membrane signals. However, because of a hint by Smith and Wells [6], it was worthwhile to investigate the presence and possible role of these peculiar phospholipids in the nucleus. Smith and Wells had concluded inositol lipid synthesis occurred in the nuclear envelope, probably contaminated by the endoplasmic reticulum. Irvine in Cambridge and Cocco in Bologna used 0.4% Triton to prepare membrane-free nuclei that were able to synthesize phosphatidyl4,5 bisphosphate (PtdIns(4,5)P2) in a cytoplasm-independent manner; this work was published in 1987 [7] and all doubts evaporated. Several follow-up papers appeared that helped the nuclear inositol lipid idea become more generally accepted, and then others also began to publish studies into nuclear phosphoinositide signalling [8, 9]. More experiments in Bologna laboratory had identified a nuclear-specific species of Phospholipase PLCβ that was activated within 2 minutes treatment of 3T3 mouse fibroblasts with insulin-like growth factor-1. These data yielded a manuscript published in Nature in 1992 [10].
At this time the persisting controversy over the presence and the role of nuclear inositol lipids and their metabolic steps was put-to-bed.
3. Nuclear inositol lipid signalling in myelodysplastic syndromes and in glioblastoma
More recently a significant body of work has been published on links between nuclear inositol lipid signalling and myelodysplastic syndromes.
A paper in the Journal of Clinical Oncology by Follo and Cocco [10] has been particularly important. They determined that mono-allelic deletion of inositide-specific Phospholipase (C PLCβ1) is associated with worse clinical outcomes in myelodysplastic syndromes. This study captured the attention of haematologists, while also paving the way for further epigenetic analysis that demonstrated how PLCβ1 mRNA levels are predictive of whether the response to azacitidine therapy will be either clinically favourable or lead to a worsening condition [11-13].
In addition to haematological diseases current work from several laboratories is focused on the possible role of abnormalities of PLCs in the pathogenesis of glioblastoma. There are hints at PLCβ1 as a novel signature gene in the molecular classification of high-grade gliomas. PLCβ1gene expression correlates with glioma’s grade, and it is lower in glioblastoma. PLCβ1 silencing in cell lines and primary astrocytes, leads to increased cell migration and invasion. These data suggest a potential role of nuclear PLCβ1 in maintaining a normal or less aggressive glioma phenotype [14, 15].
All in all the inositol lipid story is now a main field of investigation with relevant clinical aspects. Just few days ago the acknowledgment of the seminal work carried out in Bologna, which led first to the discovery and subsequently to the evidence of the role of nuclear polyphosphoinositides also in pathology, has become the subject in the journal Biomolecules of a “Honorary Special Issue Commemorating the Work of Prof. Lucio I. M. Cocco” entitled “Versatility of a Cellular Signaling Scaffold: The Inositol Ring Rules!”, edited by Professors Pavel Hozak and Stephen Shears (https://www.mdpi.com/journal/biomolecules/special_issues/0X82R285Y2)
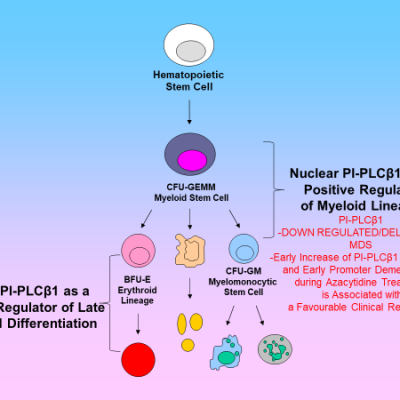

Fig. 2. Schematic picture of the role of PLCβ1 in myelodysplastic syndromes and Glioblastoma.
Conclusions
This story could appear a little provocative and perhaps aimed at demonstrating a certain provinciality of our Alma Mater. In reality it only tells true things that happened way back in the 1600s and more recently between the 70s and 2000s. The actors in this story owe a lot to Bologna and the Alma Mater. However, it is true that the most important awards have come from across the Channel, the Royal Society and from scientists far from Italy, honouring the seminal work on nuclear signalling with special publication devoted to it. At the end of the story, allow me to address a thought full of nostalgia to my mentor Francesco Antonio Manzoli, who among the many wonderful things he did, has been President and then Perpetual Secretary-Physical Sciences of the Academy of Sciences of the Bologna Institute.
References
1. West, J.B. Marcello Malpighi and the discovery of the pulmonary capillaries and alveoli. Am. J. Physiol. Lung Cell Molec. Physiol. 2013, 304, 383-390.
2. Rose, H.G.; Frenster, J.H. Composition and metabolism of lipids within repressed and active chromatin of interphase lymphocytes. Biochim. Biophys. Acta 1965, 106, 577-591. doi: 10.1016/0005-2760(65)90073-1.
3. Manzoli, F.A.; Maraldi, N.M.; Cocco, L.; Capitani, S.; Facchini, A. Chromatin phospholipids in normal and chronic lymphocytic leukemia lymphocytes. Cancer Res. 1977, 37, 843-849.
4. Cocco, L.; Maraldi, N.M.; Manzoli, F.A.; Gilmour, R.S.; Lang, A. Phospholipid interactions in rat liver nuclear matrix. Biochem. Biophys. Res. Commun. 1980, 96, 890-898. doi: 10.1016/0006-291x(80)91439-4.
5. Michell, R.H.; Kirk, C.J.; Jones, L.M.; Downes, C.P.; Creba, J.A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos. Trans. R. Soc. Lond. [Biol] 1981, 296, 123-138.
6. Smith, C.D.; Wells, W.W. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J. Biol. Chem. 1983, 258, 9368-9373.
7. Cocco, L.; Martelli, A.M.; Gilmour, R.S.; Ognibene, A.; Manzoli, F.A.; Irvine, R.F. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin-like growth factor I. Biochem. Biophys. Res. Commun. 1988, 154, 1266-1272.
8. Divecha, N.; Banfic, H.; Irvine, R.F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J 1991, 10, 3207-3214, doi: 10.1002/j.1460-2075.1991. tb04883.x.
9. Payrastre, B.; Nievers, M.; Boonstra, J.; Breton, M.; Verkleij, A.J.; Van Bergen en Henegouwen, P.M.P. A differential localization of phosphoinositide kinases, diacylglycerol kinase and phospholipase c in the nuclear matrix. J. Biol. Chem. 1992, 267, 5078-5084.
10. Martelli, A.M.; Gilmour, R.S.; Bertagnolo, V.; Neri, L.M.; Manzoli, L.; Cocco, L. Nuclear localization and signalling activity of phosphoinositidase Cb in swiss 3t3 cells. Nature 1992, 358, 242-245.
11. Follo, M.Y.; Finelli, C.; Clissa, C.; Mongiorgi, S.; Bosi, C.; Martinelli, G.; Baccarani, M.; Manzoli, L.; Martelli, A.M.; Cocco, L. Phosphoinositide-phospholipase C beta1 mono-allelic deletion is associated with myelodysplastic syndromes evolution into acute myeloid leukemia. J. Clin. Oncol. 2009, 27, 782-790. doi: 10.1200/JCO.2008.19.3748.
12. Follo, M.Y.; Finelli, C.; Mongiorgi, S.; Clissa, C.; Bosi, C.; Testoni, N.; Chiarini, F.; Ramazzotti, G.; Baccarani, M.; Martelli, A.M.; et al. Reduction of phosphoinositide-phospholipase C beta1 methylation predicts the responsiveness to azacitidine in high-risk MDS. Proc. Natl. Acad. Sci. USA 2009, 106, 16811-16816. doi: 10.1073/pnas.0907109106.
13. Cocco, L.; Finelli, C.; Mongiorgi, S.; Clissa, C.; Russo, D.; Bosi, C.; Quaranta, M.; Malagola, M.; Parisi, S.; Stanzani, M.; et al. An increased expression of PI-PLCbeta1 is associated with myeloid differentiation and a longer response to azacitidine in myelodysplastic syndromes. J. Leukoc. Biol. 2015, 98, 769-780. doi: 10.1189/jlb.2MA1114-541R.
14. Lu, G., Chang, J.T., Liu, Z., Chen, Y., Li, M., Zhu, J.J. Phospholipase C Beta 1: a Candidate Signature Gene for Proneural Subtype High-Grade Glioma. Mol. Neurobiol 2016, 53, 6511-6525. doi: 10.1007/s12035-015-9518-2.15. Ratti, S., Marvi, M.V., Mongiorgi, S., Obeng, E.O., Rusciano, I., Ramazzotti, G.; Morandi, L.; Asioli, S.; Zoli, M.; Mazzatenta, D.; Shu, P.G.; Manzoli, L.; Cocco, L. Impact of phospholipase C β1 in glioblastoma: a study on the main mechanisms of tumor aggressiveness. Cell. Mol. Life Sci. 2022, 79, 195. doi: 10.1007/s00018-022-04198-1.


